COVID-19 IgM/IgG One Step Rapid Test Kit
| Product name | SARS-CoV2(COVID-19)IgG/IgM One Step Rapid Test Kit | Test Time | 15 minutes after loading |
|---|---|---|---|
| Type | Pathological Analysis Equipments | Accuracy | 91.1% |
| Instrument classification | Class II | Format | Cassete/Strip |
| Specimen | Whole Blood/Serum/Plasma | Certificate | ISO9001/13485 |
| Specification | 3.00mm/4.00mm | Shelf Life | 2 years |
| Storage Temperature | 2-30°C | Packing | 40 tests/box, 65 box/carton, 660*360*565mm G.W:22KG/carton |
| Total Combined (IgG+IgM) | Early Stage (IgG+IgM) | Mid-late Stage (IgG+IgM) | IgM | IgG | |
|---|---|---|---|---|---|
| Total Confirmed Cases (by PCR) | 203 | 58 | 143 | 203 | 203 |
| Positive | 185 | 44 | 140 | 165 | 170 |
| Negative | 18 | 14 | 3 | 38 | 33 |
| Sensitivity | 91.1% | 75.9% | 97.9% | 81.3% | 83.7% |
The One Step SARS-CoV2(COVID-19)IgG/IgM Test is a rapid chromatographic immunoassay for the qualitative detection of antibodies (IgG and IgM) to COVID-19 virus in Whole Blood /Serum / Plasma to aid in the diagnosis of COVID-19 viral infection.
Corona viruses are enveloped RNA viruses that are distributed broadly among humans, other mammals, and birds and that cause respiratory, enteric, hepatic and neurologic diseases. Seven corona virus species are known to cause human disease. Four viruses-229E. OC43. NL63 and HKu1- are prevalent and typically cause common cold symptoms in immunocompetent individuals.4 The three other strains-severe acute respiratory syndrome coronavirus (SARS-Cov), Middle East respiratory syndrome coronavirus (MERS-Cov) and 2019 Novel Coronavirus (COVID-19)- are zoonotic in origin and have been linked to sometimes fatal illness. IgG and lgM antibodies to 2019 Novel Coronavirus can be detected with 2-3 weeks after exposure. lgG remains positive, but the antibody level drops overtime.
The One Step SARS-CoV2(COVID-19)IgG/IgM (Whole Blood/Serum/Plasma) is a lateral flow immunochromatographic assay. The test uses anti-human lgM antibody (test line IgM), anti-human lgG(test line lgG and goat anti-rabbit igG (control line C) immobilised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to recombinant COVID-19 antigens conjugated with colloid gold (COVID-19 conjugatesand rabbit lgG-gold conjugates. When a specimen followed by assay buffer is added to the sample well, IgM &/or lgG antibodies if present, will bind to COVID-19 conjugates making antigen antibodies complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the corresponding immobilized antibody (anti-human IgM &/or anit-human lgG) the complex is trapped forming a burgundy colored band which confirm a reactive test result. Absence of colored band in the test region indicates a non-reactive test result.
The test contains an internal control(C band)which should exhibit a burgundy colored band of the immunocomplex goat anti rabbit IgG/rabbit lgG-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
Store as packaged in the sealed pouch at room temperature or refrigerated (4-30℃ or 40-86℉). The test device is stable through the expiration date printed on the sealed pouch.
The test must remain in the sealed pouch until use.
Serology tests have been adopted as one of the lab confirmation tests (molecular PCR, sequencing and serology) of COVID-19, published on the Guideline for COVID-19 Diagnosis and Treatment, 7th Editionby the National Health Commission of RR.China dated March 3rd, 2020. "Seroconversion of COVID-19 specific IgM antibodies usually can be detected in 3-5 days after symptoms"
| Test devices | Disposable specimen droppers |
| Buffer | Package insert |
| Centrifuge | Timer |
| Alcohol Pad | Specimen collection containers |
☆ For professional in vitro diagnostic use only. Do not use after expiration date.
☆ Do not eat, drink or smoke in the area where the specimens and kits are handled.
☆ Handle all specimens as if they contain infectious agents.
☆ Observe established precautions against microbiological hazards throughout all procedures and follow the standard procedures for proper disposal of specimens.
☆ Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
☆ Follow standard bio-safety guidelines for handling and disposal of potential infective material.
☆ Humidity and temperature can adversely affect results.
1. The SARS-CoV2(COVID-19)IgG/IgM Test can be performed used on Whole Blood /Serum / Plasma.
2. To collect whole blood, serum or plasma specimens following regular clinical laboratory procedures.
3. Testing should be performed immediately after specimen collection. Do not leave the specimens at room temperature for prolonged periods. For long term storage, specimens should be kept below -20℃. Whole blood should be stored at 2-8℃ if the test is to be run within 2 days of collection. Do not freeze whole blood specimens.
4. Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
1. Allow the test, specimen, buffer and/or controls to reach room temperature 15-30℃ (59-86℉) prior to testing.
2. Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible.
3. Place the test device on a clean and level surface.
4. Hold the dropper vertically and transfer 1 drop of specimen (approximately 10μl) to the specimen well(S) of the test device, then add 2 drops of buffer (approximately 70μl) and start the timer. See illustration below.
5. Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes.
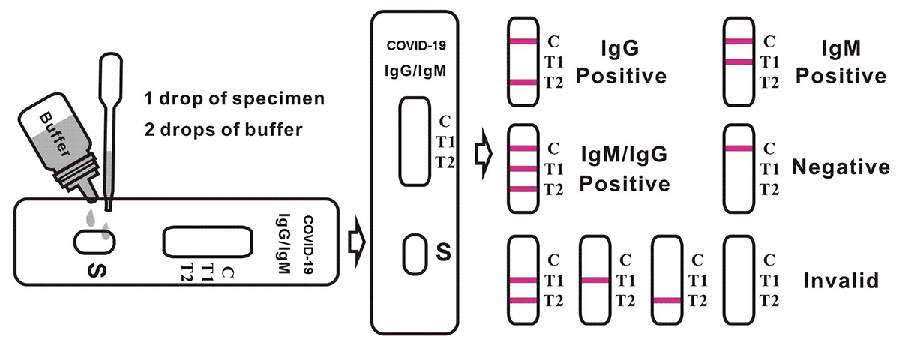
Applying sufficient amount of specimen is essential for a valid test result. If migration (the wetting of membrane) is not observed in the test window after one minute, add one more drop of buffer to the specimen well.
Positive: Control line and at least one test line appear on the membrane. The appearance of T2 test line indicates the presence of COVID-19 specific IgG antibodies. The appearance of T1 test line indicates the presence of COVID-19 specific IgM antibodies. And if both T1 and T2 line appear, it indicates that the presence of both COVID-19 specific IgG and IgM antibodies. The lower the antibody concentration is, the weaker the result line is.
Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
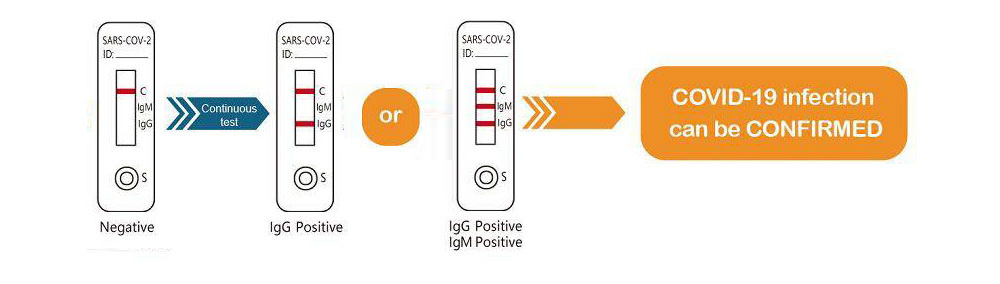
1. The SARS-CoV2(COVID-19)IgG/IgM Test is for in vitro diagnostic use only. The test should be used for the detection of COVID-19 antibodies in Whole Blood /Serum / Plasma specimens only. Neither the quantitative value nor the rate of increase in
2. COVID-19 antibodies can be determined by this qualitative test.
3. As with all diagnostic tests, all results must be interpreted together with other clinical information available to the physician.
4. If the test result is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not at any time preclude the possibility of COVID-19 viral infection.

1.Prepare

2.Cover

3.Cross membrane

4.Cut strip

5.Assembly

6.Pack the pouches

7.Seal the pouches

8.Pack the box

9.Encasement


